Veterinaria (Montevideo), 2025 61, (224), 10.29155/VET.61.224.6
Artículo original
Evaluation of potential kidney damage associated with the use of phenobarbital and potassium bromide in epileptic canines using biomarkers predicting early damage
Evaluación del potencial daño renal asociado al uso de fenobarbital y bromuro de potasio en caninos epilépticos mediante el empleo de biomarcadores predictores de daño precoz
Avaliação do potencial de dano renal associado ao uso de fenobarbital e brometo de potássio em cães epilépticos mediante o uso de biomarcadores preditivos de lesão renal precoce
Clarisa Brighenti¹ https://orcid.org/0009-0008-7516-0208
Alejandra Capelli² https://orcid.org/0000-0003-1388-0345
Walter Norbis3 https://orcid.org/0000-0002-7819-3450
Claudia Della Cella¹ https://orcid.org/0000-0002-2387-2595
Luis Delucchi¹ https://orcid.org/0000-0001-9659-228X
Catherine Fagúndez¹ https://orcid.org/0000-0002-7315-1438
¹ Departamento de Clínica y Hospital Veterinario, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay. Corresponding author: cfagundez19@gmail.com
2 Departamento de Patobiología, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay.
3 Departamento de Biología Animal, Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay.
Abstract
Epilepsy is a neurological disease present in a wide range of mammals, including humans and domestic canines and felines. The most commonly used pharmacological treatments for canines are phenobarbital and potassium bromide (KBr). However, both of these medications are partially eliminated through the kidneys, which could potentially cause damage at this level. Current diagnostic methods for kidney damage typically identify issues only at advanced stages, highlighting the need for early detection techniques. Thus, this study aims to evaluate early renal alterations in canines diagnosed with idiopathic epilepsy and treated with phenobarbital and/or KBr, using specific biomarkers such as kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), alongside non-specific biomarkers such as C-reactive protein (C-RP). Data from 39 canines treated at the Veterinary Hospital Centre (Udelar) and private clinics in Montevideo, Uruguay, were analysed. Subjects were categorised into two groups: a control group (healthy animals) and a group of subjects previously diagnosed with idiopathic epilepsy. The latter group was subdivided into three additional groups: one treated with phenobarbital, one treated with phenobarbital/KBr, and one treated with KBr alone. Laboratory results were evaluated for new biomarkers and conventional analytes. No alterations were found in laboratory tests for liver function, profiles, or urinalysis among the 30 canines subjected to the different treatments. Regarding specific biomarkers, no statistically significant differences (p > 0.05) were observed among the groups. However, alterations in C-RP were found in the group treated only with KBr. Under the conditions of the trial, no significant changes were detected in urinalysis or renal profiles associated with the administration of phenobarbital and/or potassium bromide in canines with idiopathic epilepsy.
Keywords: Early diagnostic methods, New kidney biomarkers, Antiepileptic treatments.
Resumen
La epilepsia es una enfermedad neurológica presente en casi todos los mamíferos, incluidos humanos, caninos y felinos. Los tratamientos farmacológicos más utilizados para los caninos son el fenobarbital y el bromuro de potasio (KBr). Sin embargo, ambos medicamentos son eliminados en parte por la vía renal, lo que potencialmente podría provocar daño a este nivel. Los métodos de diagnóstico convencionales utilizados para detectar daño renal identifican problemas en etapas avanzadas, lo que remarca la necesidad de técnicas de detección temprana. Este estudio tiene como objetivo evaluar las alteraciones renales tempranas en caninos diagnosticados con epilepsia idiopática y tratados con fenobarbital y/o KBr, utilizando biomarcadores específicos, como Kidney Injury Molecule-1 (KIM-1) y Neutrophil Gelatinase-Associated Lipocalin (NGAL), junto con biomarcadores no específicos, como la proteína C reactiva (PCR). Se analizaron datos de treinta y nueve caninos atendidos en el Centro Hospitalario Veterinario (Udelar) y clínicas privadas de Montevideo, Uruguay. Los animales se clasificaron en dos grandes grupos: uno de control (animales sanos) y otro de animales previamente diagnosticados con epilepsia idiopática. Este último se subdividió en tres grupos: tratamiento con fenobarbital, tratamiento con fenobarbital/KBr y tratamiento con KBr. Se evaluaron los resultados de laboratorio de los nuevos biomarcadores y de analitos convencionales. No se encontraron alteraciones en pruebas de laboratorio de función hepática, perfiles renal y análisis de orina para los treinta caninos sometidos a los diferentes tratamientos. En cuanto a los biomarcadores específicos, no se observaron diferencias estadísticamente significativas (p > 0,05) entre los grupos. Sin embargo, se encontraron alteraciones en la proteína C reactiva en el grupo tratado solo con KBr. En resumen, bajo las condiciones del ensayo, no se detectaron cambios significativos en el análisis de orina ni en los perfiles renales asociados con la administración de fenobarbital y/o bromuro de potasio en caninos con epilepsia idiopática.
Palabras clave: Métodos de diagnóstico temprano, Nuevos biomarcadores renales, Tratamientos antiepilépticos.
Resumo
A epilepsia é uma doença neurológica presente em uma ampla gama de mamíferos, incluindo humanos, caninos e felinos domésticos. Os tratamentos farmacologicos mais comumente utilizados para caninos são o fenobarbital e brometo de potássio (KBr). No entanto, ambos os medicamentos são parcialmente eliminados pelos rins, o que pode causar danos nesse nível. Os métodos atuais de diagnóstico de danos renais normalmente identificam problemas apenas em estágios avançados, destacando a necessidade de técnicas de detecção precoce. Assim, este estudo tem como objetivo avaliar alterações renais precoces em caninos com diagnóstico de epilepsia idiopática e tratados com fenobarbital e/ou KBr, utilizando biomarcadores específicos como Kidney Injury Molecule-1 (KIM-1) e Neutrophil Gelatinase-Associated Lipocalin (NGAL), juntamente com biomarcadores inespecíficos, como a proteína C reativa (PCR). Foram analisados dados de 39 caninos atendidos no Centro Hospitalar Veterinário (Udelar) e clínicas privadas de Montevidéu, Uruguai. Os indivíduos foram categorizados em dois grupos, um grupo controle (animais saudáveis) e um grupo de indivíduos previamente diagnosticados com epilepsia idiopática. Este último grupo foi subdividido em outros três grupos, um grupo tratado com fenobarbital, um grupo tratado com fenobarbital/KBr e um grupo tratado apenas com KBr. Os resultados laboratoriais de novos biomarcadores e analitos convencionais foram avaliados. Não foram encontradas alterações nos exames laboratoriais de função hepática, perfis ou urinálise entre os trinta caninos submetidos aos diferentes tratamentos. Em relação aos biomarcadores específicos, não foram observadas diferenças estatisticamente significativas (p > 0,05) entre os grupos. Porém, foram encontradas alterações na PCR no grupo tratado apenas com KBr. Em resumo, nas condições do ensaio, não foram detectadas alterações significativas no exame de urina ou nos perfis renais associados à administração de fenobarbital e/ou brometo de potássio em caninos com epilepsia idiopática.
Palavras-chave: Métodos de diagnóstico precoce, Biomarcadores de notícias renais, Tratamentos antiepilépticos.
Fecha de recibido: 29/01/2025
Fecha de aceptado: 01/10/2025
1. Introduction
Epilepsy is a neurological disorder that affects nearly all mammals, including humans. Among domestic animals, canines and felines are the most affected. Currently, there is no definitive cure or treatment for epilepsy (Podell, 2013). The first-line pharmacological treatments for canines are phenobarbital (PB) and potassium bromide (KBr), chosen for their extensive historical knowledge, global availability, and low cost (Bhatti et al., 2015). However, these medications cause several adverse effects on different organs and systems, which are still under study. Therefore, the use of these drugs can alter normal values of several biomarkers, making them useful diagnostic methods for assessing biological processes resulting from these treatments (Joyce et al., 1985; Metairon et al., 2009).
Biomarkers related to liver function damage, such as alkaline phosphatase (ALP), have been reported as altered due to prolonged use of PB (Baird-Heinz et al., 2012). The drug is excreted unchanged through the kidneys (approximately 25 %). On the other hand, the use of PB is considered relatively safe, since reports of tissue or organ damage are rare (Bhatti et al., 2015; Joyce et al., 1985). On the other hand, KBr is primarily eliminated through the kidneys, with some reports of kidney problems associated with its use in humans (Joyce et al., 1985; Metairon et al., 2009). Moreover, Van Logten et al. (1973) reported that rats receiving dietary supplementation with sodium bromide showed renal enlargement upon necropsy and elevated concentrations of bromide (Br-) in their kidneys. However, they did not exhibit signs of nephrotoxicity. Yokota et al. (2017) also reported an exacerbation of glomerular lesions and increased expression of kidney injury markers associated with KBr consumption. Prolonged use of PB and KBr can significantly alter laboratory test results, leading to variations in concentrations of specific and non-specific biomarkers (Bhatti et al., 2015; Goiz-Márquez et al., 2008; Muñana, 2013).
Conventional blood diagnostic methods used to assess kidney function have limitations, often leading to late diagnosis and a poor prognosis (Perini et al., 2021). This highlights the need to find new biomarkers for early diagnosis. Detecting the disease at an initial stage can increase the chances of effective treatment, improve prognosis, enable preventive measures, allow assessment of disease progression, and ultimately enhance the patient’s quality of life (Perini et al., 2021).
Alternatives for early diagnosis of kidney disease have been sought using analytes associated with the progression of kidney deterioration. A clear example is the determination of symmetrical dimethylarginine (SDMA), which is a specific marker for early kidney damage (Kielstein et al., 2006). SDMA is a methylated form of arginine present in all nucleated cells. Following proteolysis and kidney excretion, it is released into the bloodstream. It is related to the glomerular filtration rate (GFR) in humans, dogs, and cats and can be measured in serum or plasma (Han et al., 2002; Mishra et al., 2005; Soni et al., 2009). The SDMA concentration increases when the glomerular filtration rate decreases between 25 % and 40 %. This biomarker appears to be more specific than serum creatinine, since it is not influenced by extrarenal factors like body condition and advanced age. Furthermore, SDMA levels rise before serum creatinine, allowing for earlier diagnosis (Ernst et al., 2018; Harjen et al., 2021).
Recent studies have reported that the biomarkers kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) may be associated with early renal failure (Han et al., 2002; Mishra et al., 2005; Soni et al., 2009). KIM-1 is a type I membrane glycoprotein from the immunoglobulin superfamily, expressed in the apical region of epithelial cells in damaged renal tubules, as its expression is low in healthy kidneys (Ichimura et al., 2008; Lippi et al., 2018; Nabity & Hokamp, 2023; Reyes-Uribe et al., 2022). NGAL is a low-molecular-weight glycoprotein that is covalently linked to a neutrophil gelatinase and is synthesised by renal tubular cells. An increase in NGAL levels indicates renal tubular epithelial injury (Miguel et al., 2021; Segev et al., 2013).
The primary route of elimination for KBr is renal. It is commonly used in chronic patients who already experience a reduced quality of life due to epilepsy. This study aimed to determine whether early renal alterations occur in epileptic canines treated with phenobarbital and/or potassium bromide, either as monotherapy or in combination. Assessment was conducted using specific and non-specific biomarkers such as NGAL and KIM-1 (Bhatti et al., 2015; Metairon et al., 2009; Potschka et al., 2015; Thomas, 2000).
2. Materials and methods
This study was conducted at the Faculty of Veterinary in the Small Animal Clinic and Pathology Unit at the University of the Republic, under a protocol approved by the Honorary Commission for Animal Experimentation (CEUA FVET Protocol-form 1397/111900-000673-21). This research was carried out with the signed consent of the animals’ owners from November 2021 to December 2022.
2.1. Subjects
This study involved 39 total canines: 30 (Canis lupus familiaris) from the Neurology Clinic at the Veterinary Hospital Centre of the Faculty of Veterinary and from private veterinary clinics, as well as nine healthy animals (controls) from private owners. Based on the total number of animals, four study groups were established: control group (G1), consisting of 9 healthy canines; Group 2 (G2), with 10 canines treated only with phenobarbital; Group 3 (G3), with 10 canines receiving a combination of phenobarbital and potassium bromide; and Group 4 (G4), with 10 canines treated only with potassium bromide. The animals in G3 and G4 were treated with KBr for a minimum of 3 months to reach baseline serum concentrations. Moreover, animals from G2 and G3 were treated with PB for at least 15-30 days to reach the baseline serum concentration. Among the 39 canines, 18 were female and 21 were male. Additionally, 27 were neutered and 12 were intact; 24 were purebred and 15 were mixed breeds; their ages ranged from 1 to 13 years. The average treatment durations for the different groups were as follows: G2 (phenobarbital only) had an average of 27.80 months (range: 6 to 84 months); G4 (potassium bromide only) had 37.80 months (range: 6 to 144 months); G3 (phenobarbital and potassium bromide) had 44.70 months (range: 17 to 96 months); and G4 potassium bromide alone had 36.20 months (range: 14 to 96 months). Inclusion criteria did not consider sex, breed, age, or size for each group. In G1, the animals were clinically healthy, without any pathologies or pharmacological treatments. All individuals included in G2, G3, and G4 had idiopathic epilepsy.
Exclusion criteria for G1 included having any alteration in the general and/or neurological objective examination, as well as blood and/or urinary parameters outside the normal range, except for eosinophils. In G2, G3, and G4, exclusion criteria were animals that received treatment other than what was assigned to their respective groups, had other concomitant diseases, or presented a renal profile outside of established ranges.
2.2. Clinical examination
Prior to inclusion in the protocol, all animals underwent individual physical examinations, a general objective examination (GOE), and a neurological examination. Additionally, paraclinical tests were performed by collecting and extracting a single urine and blood sample at the beginning of the study. These included a complete blood count, liver function tests, a renal profile (urea and creatinine), and a physical and chemical urine analysis to assess their health status. The complete blood count, biochemistry (liver function and renal profile), urinalysis, and renal biomarker tests were performed in the Clinical Analysis, Endocrinology, and Animal Metabolism Laboratory of the Faculty of Veterinary.
The blood counts were performed on an automated counter (Mythic 18 Vet Hematology Analyzer, Orphée), and a blood smear was taken from each sample simultaneously for leukocyte differentiation and stained with May-Grünwald Giemsa stain. Regarding serum biochemistry, a liver function test was performed to determine albumin, globulins, total protein, cholesterol, total bilirubin, serum alkaline phosphatase (SAP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST), and a renal profile was performed to determine urea and creatinine levels. These parameters were measured using a semi-automatic CB 350i (Wiener LabGroup) with reagents supplied by the company. Wiener LabGroup quality controls were used, with a coefficient of variation of less than 10 % for all variables. Urine analysis was performed through physical and chemical analysis, as well as sediment evaluation. For chemical analysis, urine reagent strips (URIT 10G) were used, and the results were quantified using the URIT-50 equipment. For sediment evaluation, the urine sample was centrifuged at 1500 rpm for 5 to 10 minutes, and the resulting sediment was observed under an optical microscope at 40X and 100X magnification. Urine specific gravity was also determined using a manual clinical refractometer, and proteinuria was quantified using a CB350i semi-automated system (Wiener Lab) with the company’s dedicated reagents. Quality controls from the Wiener Lab Group, with a coefficient of variation less than 10 %, were used to determine protein in urine.
2.3. Serum determination of PB, KBr, KIM-1, NGAL, and C-RP
The serum determination of KBr was carried out using a spectrophotometric method developed and validated by Robaina et al. (2020). On the other hand, the method used to determine PB in serum was a fluorescence polarisation immunoassay (Lu-Steffes et al., 1982). One-time serum concentrations of KBr and PB were determined for G2, G3, and G4 in the Pharmacology and Therapeutics Laboratory of the Faculty of Veterinary.
To determine the serum concentration of the biomarkers KIM-1, NGAL, and C-RP, a previously reported ELISA kit for canines (My BioSource.com Antibody-Protein-Elisa Kit) was used (Wasung et al., 2015).
2.4. Statistical analysis
After verifying the assumptions of normality using the Shapiro-Wilk test and homogeneity of variances with Levene’s test (Zar, 2010), parametric analyses of variance (ANOVA) were conducted to compare variables among the groups. For non-normally distributed data, Levene’s test with the median criterion was applied. If assumptions were not met, the non-parametric Kruskal-Wallis test was used. When significant differences were detected among groups, post hoc multiple comparisons were conducted using the Tukey test (for parametric analysis) or the Mann-Whitney test with Bonferroni correction (for non-parametric analysis). Additionally, linear regression models analysed the relationships between treatment time and variables such as ALP, ALT, haemoglobin, KIM-1, NGAL, and C-RP, as well as between drug concentration in serum and several biomarkers (KIM-1, NGAL, C-RP) (Hammer et al., 2001). A significance level of p = 0.05 was used for all analyses. All statistical analyses were conducted using the PAST statistical program (Version 4.03) (Hammer et al., 2001). To determine whether the number of individuals with urine specific gravity values outside the reference ranges (both high and low) was significantly different from what would be expected under normal conditions, data from all 39 animals were evaluated. A chi-square goodness-of-fit test was used to compare observed frequencies with expected frequencies.
3. Results
3.1. Physical examination: GOE and neurological
During the physical examination, G1 showed no alterations. In contrast, G2, G3, and G4 contained some animals with uncontrolled epileptic seizures (9/30). Furthermore, these last groups (G2, G3, and G4) presented alterations in both examinations: two animals from G2, one from G3, and three others from G4. They exhibited alterations in the GOE, specifically in muscle tone and tropism. They also exhibited sensory abnormalities and unusual movements, which coincided with the previously cited reports. Moreover, these patients (n = 6) presented alterations in the neurological examination, including changes in mental status, muscular condition, gait, cranial and spinal nerves, as well as proprioception tests.
3.2. Hemogram
The parameters analysed for all animals were within normal ranges, except for eosinophil values, which were elevated in some canines that were not dewormed (19/39). Additionally, one canine presented leucocytosis with neutrophilia (1/39).
3.3. Renal profile and urine test
Regarding the renal profile (urea and creatinine), all values obtained were within the normal range.
For urine analysis, urine density was considered the most relevant data. Of the 39 animals evaluated, 28 presented values within the reference ranges, while 11 showed alterations: Five animals presented values above the range (1 belonging to G1 and 4 to G2), and six animals presented values below (1 corresponding to G2, 2 to G3, and 3 to G4). Regarding the presence of protein in urine, all groups remained within the established reference values. In total, 11 animals (28.2 %) had urinary density outside the reference range. The chi-square statistic (χ²) was 42.7, with 1 degree of freedom (df) and a p-value < 0.0001. This result indicates that the number of animals with urine density outside the reference range is statistically significant, being greater than what would be expected by chance. In this case, the number of animals outside the reference range (11) was analysed compared to what would be expected by chance (2); exact urine specific gravity values were not used. That is, the distribution of animals within and outside the reference range was evaluated to determine whether the difference between what was observed and what was expected was statistically significant. It should be noted that the reference values used for comparison were obtained from the literature.
The four study groups were divided into three age ranges: 1-5 years, 6-10 years, and over 11 years (Table 1). Young individuals predominated in G1 and G2. Group G3 presented a balanced distribution among the three age categories. Group G4 was characterised by a higher proportion of adult canines (n = 7), followed by two individuals over 11 years of age and a single canine in the 6-10 age range (Table 1).
Table 1. Distribution of canines by group and age range

3.4. Biomarker studies: NGAL, KIM-1, and C-RP
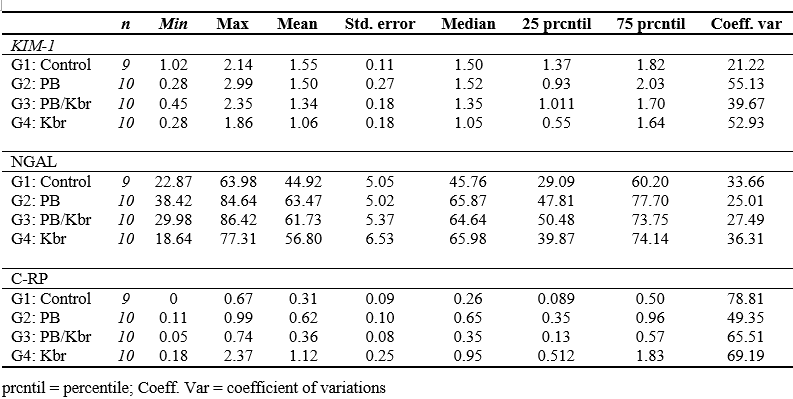
A trend toward decreased KIM-1 means was observed in the treatment groups compared to the control group, although this difference did not reach statistical significance. Additionally, the standard deviation was lower in the control group compared to the different treatments (Table 2). The NGAL means in the treated groups showed an upward trend compared to the control group, without this variation being statistically significant, with a similar pattern observed in the median. The standard deviation was higher in the control group and the KBr group (Table 2).
The C-RP averages were higher in the treatment groups vs the control group, and the median showed a similar pattern. Finally, the standard deviation was higher in G4 compared to the control and the other groups (Table 2).
Table 2. Descriptive statistics of biomarkers for G1, G2, G3 and G4 in ng/mL. KIM-1: Kidney Injury Molecule; NGAL: Neutrophil Gelatinase-Associated Lipocalin; C-RP: C - reactive protein. Given the inexistence of standardised values for KIM-1 and NGAL, the animals from the control group were used as reference values. (Reference range for KIM-1: 1.016-2.139 ng/mL; Reference range for NGAL: 22.867-63.978 ng/mL and reference range for C-RP: 0.000-0.686 ng/mL).
3.5. Biomarkers of liver function
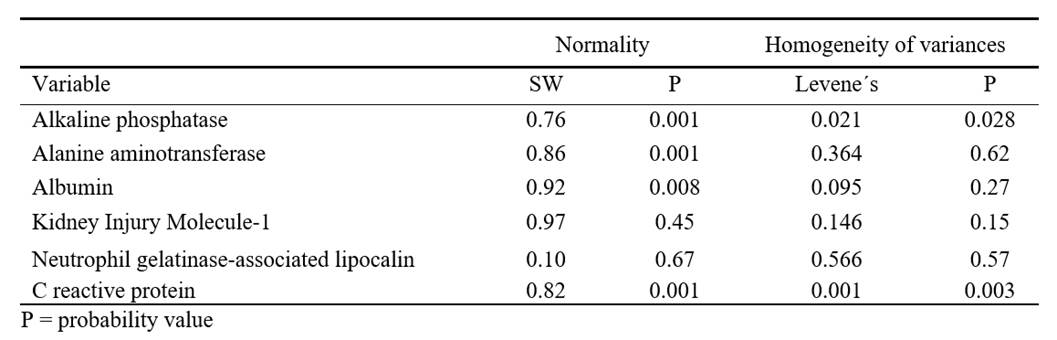
The means and medians for albumin were similar between the control and treatment groups, with minimal variation in standard deviations. For alanine aminotransferase (ALT), both the means and medians were higher and more variable compared to the control group. Treatment averages and medians for ALT in G2 were higher than those in the control group, with a higher standard deviation (Table 3). The distribution of ALP, ALT, Albumin, and C-RP was not normal. Furthermore, ALP and C-RP did not exhibit homogeneity of variances (Table 4).
Table 3. Descriptive statistics of Albumin, GPT and Alkaline Phosphatase for the test animals. VR: Reference value; Albumin VR:3.1 - 4.2 g/dl; GPT: ALT VR:20 - 98 U/l; Alkaline Phosphatase VR:17 - 111 U/l.
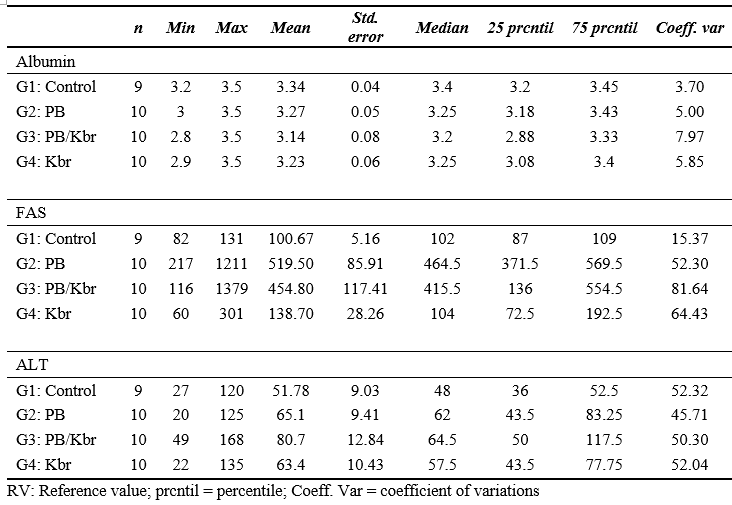
Table 4. Results of the Shapiro - Wilk (SW) normality test and homogeneity of variances (Levene’s) tests for the variables analysed
Significant differences were found among the groups for ALP (KW = 24.08; p < 0.05), specifically between the control group and G2 and G3. Additionally, significant differences were observed among the groups for C-RP values (KW: H = 12.43; p = 0.006 < 0.05) (Table 5). This difference was noted between the group treated with potassium bromide (G4) and the control group (G1), indicating an increase in C-RP levels in G4 (Figure 1).
Table 5. Results of the analysis of variance and Kruskall Wallis analysis for each variable analysed (hepatic functional and biomarkers) and the different pharmacological treatments (phenobarbital; phenobarbital/potassium bromide and potassium bromide) in canines with epilepsy
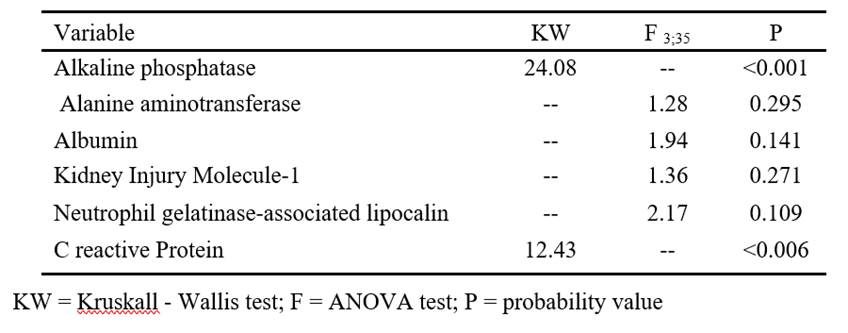
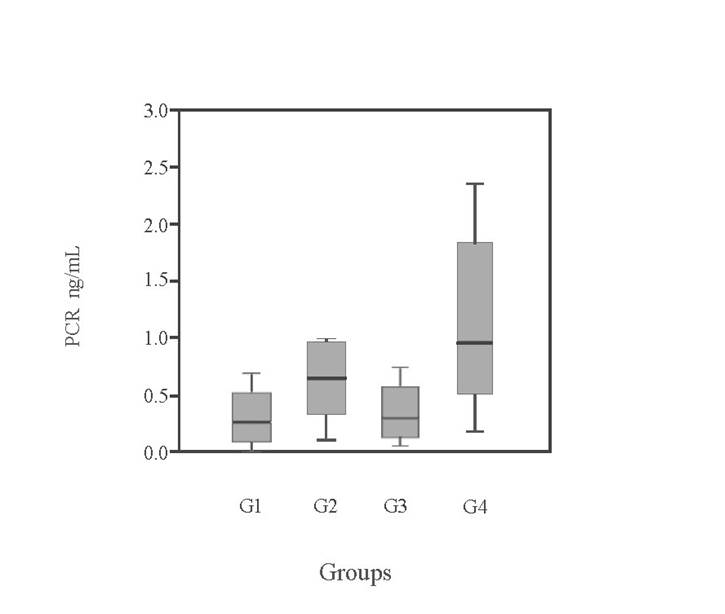
Figure 1: Ranges of serum levels of C-reactive protein (C-RP) in the four experimental groups expressed in ng/mL (control group [G1]; phenobarbital alone [G2]; phenobarbital and potassium bromide [G3] and potassium bromide alone [G4])
To assess potential drug toxicity over time, the relationship between treatment duration and the variables ALP, ALT, albumin, KIM-1, NGAL, and C-RP, as well as between serum drug concentrations and biomarkers (KIM-1, NGAL, and C-RP), was analyzed. The results showed no significant relationships between treatment duration and any of the variables analyzed (p > 0.05). Likewise, no relationship was found between serum drug concentrations and the biomarkers evaluated.
4. Discussion
In the present study, some of the animals that did not achieve seizure control exhibited abnormalities in both neurological and general physical examinations, as has been previously reported (Feijoo et al., 2020). Among the patients diagnosed with idiopathic epilepsy in our sample 80 % had a normal physical examination, which is consistent with published literature. Armaşu et al. (2014) reported that among 258 canines diagnosed with idiopathic epilepsy, 84 % did not show any abnormalities in neurological examination. However, in cases of epilepsy caused by symmetrical and asymmetrical brain lesions, neurological examination abnormalities were found in 55 % and 47 % of cases, respectively.
Regarding the haematological results, animals with eosinophil values exceeding the reference range (which were not dewormed) exhibited a potential cause for this deviation, as noted in the literature (Noemi, 1999; Rebar, 2003). Additionally, elevated eosinophil values may also result from moderate hypersensitivity reactions (Rebar, 2003). Among the 39 animals included in this trial, only one presented leucocytosis with neutrophilia without an apparent cause; this finding could be attributed to various causes. The primary function of neutrophils is to maintain health by combating bacterial or viral infections, including acting in parasitosis, sepsis, acute inflammatory states, and extensive tissue destruction. Thus, an increase in neutrophils in the bloodstream could be an indicator of non-specific systemic infections (Rebar, 2003).
The administration of phenobarbital and/or potassium bromide did not cause alterations in urine test results or renal profile. Regarding the renal profile, the determination of urea, creatinine, and urinary density values is a useful tool in evaluating renal function. Plasma creatinine and urea concentrations can also provide an estimate of the glomerular filtration rate. Elevated creatinine values suggest that kidney damage is fairly advanced, with serious implications for the patient’s quality of life. In this study, none of the animals were at this stage, as their results were within normal limits and ranges (Ernst et al., 2018).
Some deviations from normal urine density values were observed, which were associated with changes in diet or the amount of water ingested. The owners indicated that the five patients with high urine specific gravity were fasted from solid food and water, while the six patients with low urinary density drank more water than usual, and their samples were not the first urine of the morning. Urine is composed of 95 % water and 5% soluble organic and inorganic substances and ions; the variation in composition is largely determined by nutrition and the time of day at which the sample is taken (Di Bartola & Westropp, 2020). The significant differences in the results suggest the possibility of underlying factors (environmental, pathological, nutritional, water consumption, and time of sample collection) that could be affecting the urine specific gravity of the animals tested (Di Bartola & Westropp, 2020).
Renal azotemia begins when three-quarters of the nephrons are affected, making it a key indicator of renal failure. Regardless of the type of kidney pathology, whether acute or chronic, urinary density will always be low. This parameter is influenced by factors such as body weight, diet, exercise, age, weather conditions, metabolism, and hydration status. The normal range for urinary density is between 1.015 and 1.045. However, these results should be interpreted in conjunction with the animal’s hydration status and recent water intake. Therefore, a single measurement outside these limits does not necessarily indicate renal impairment.
No significant differences were detected in the plasma concentration values of KIM-1 and NGAL compared to the control group, indicating the absence of early kidney disease. Although renal failure associated with the administration of potassium bromide has been reported in both humans and rats, there is no scientific evidence that this occurs in canines (Joyce et al., 1985; Metairon et al., 2009; Van Logten et al., 1973). Specific biomarkers, such as KIM-1 and NGAL, are used to detect tubular damage, and any signs of alterations at this level were sought in the study. However, it should be noted that no standardised reference values have been established for these biomarkers in either humans or animals, making their interpretation challenging, particularly in absolute terms. Zheng et al. (2019) reported serum concentration values for KIM-1 < 48.52 pg/mL and NGAL < 25.31 ng/mL in healthy animals. The values obtained in our trial do not align with those reported by Zheng et al. (2019). This discrepancy may be attributed to differences in statistical design, as our analysis involved a limited number of cases with high variability. Zheng et al. (2019) used 16 beagle dogs weighing 5.1 ± 0.2 kg, which were housed under uniform conditions for 4 weeks, with consistent feeding and handling regimes throughout the experiment. In contrast, the animals in our study ranged in age from 1 to 13 years across all groups, with varied housing conditions, management practices, and durations of medication use (ranging from 6 months to 96 months for PB and from 6 months to 144 months for KBr). This variability is because our study was based on patients with private owners at the Faculty of Veterinary clinic and private clinics.
We considered that it would have been appropriate to use untreated epileptic canines as a reference group, considering the new biomarkers, which would allow us to determine whether possible variations in the levels of these molecules are actually due to the treatment used and not to the epilepsy itself. However, this presents important limitations in the number of patients, since untreated epilepsy cases that reach the clinic are rare, so at the beginning of this trial it was decided not to include a group with these characteristics. However, observing the results obtained, we can consider that there should be no differences between untreated and treated epileptic animals, since no significant differences were found in the concentrations of the study biomarkers between the control group and groups 2, 3, and 4.
Advanced age (>6 years), weight, body condition, and dietary differences have been identified as contributing risk factors for the development of chronic kidney disease (Pérez-Sánchez et al., 2023). Furthermore, age and the different diets consumed by animals can cause variations in serum profiles, mainly related to renal and hepatic ageing processes (Perini et al., 2021). In the present study, these factors, along with variability in the timing of drug administration, could have influenced the results obtained.
Phenobarbital, used either as monotherapy or in combination, led to a significant increase in serum ALP and C-RP levels. The most relevant findings of this study were the serum concentrations of ALP and C-RP in animals under chronic administration of these drugs, compared to the control group. Significant differences were found between the control group (G1) and G4 in the serum concentration of C-RP, a general biomarker. This can be explained by several factors, as C-RP is a positive biomarker considered non-specific, with increased concentrations associated with damage due to various pathologies (Caspi et al., 1987). The average C-RP concentration in the control group (G1) was 310 μg/mL, within a range of 0-690 μg/mL, consistent with Caspi et al. (1987), who reported normal values of < 5,000 μg/mL. However, discrepancies exist in the literature regarding these values. Therefore, the results obtained for the control group are used as reference values. Within G4, one patient with a C-RP concentration of 1,140 μg/mL also presented leucocytosis with neutrophilia, which aligns with findings from various authors who have linked elevated C-RP levels to infectious and inflammatory processes (Parra et al., 2005). The physical examination of this animal was normal, with no clinical evidence of any underlying pathology. Except for idiopathic epilepsy, no other clinical conditions were observed that could justify an increase in these values.
Regarding ALP, several authors have reported that the chronic use of phenobarbital can lead to increased levels of this enzyme, as well as ALT (Goiz-Márquez et al., 2008; Muñana, 2013; Thomas, 2000). However, no significant differences in ALT levels were found among the groups, nor were there deviations from the reference values, although G2 and G3 did show significant differences compared to G1 and G4. Additionally, it was not possible to correlate any biomarker with the duration of treatment, likely due to the considerable variability in treatment times and the small sample size. Notably, G4, which received only potassium bromide, did not show the expected altered ALP values as reported in the literature (Goiz-Márquez et al., 2008; Metairon et al., 2009; Muñana, 2013).
Thus, no significant correlations were found between biomarker concentrations, likely due to the small sample size. Additionally, no significant relationship was identified between specific biomarker values and serum concentrations of anticonvulsant drugs. It was also not possible to determine whether there is a relationship between treatment duration and biomarker concentrations, suggesting that there may be no early kidney damage associated with the drugs implemented in this study.
5. Conclusion
From this work, we can conclude that early renal alterations could not be detected through the biomarkers studied, KIM-1 and NGAL, in canines treated with phenobarbital and/or potassium bromide, whether used as monotherapy or in combination.
References
Armaşu, M., Packer, R., Cook, S., Solcan, G., & Volk, H. (2014). An exploratory study using a statistical approach as a platform for clinical reasoning in canine epilepsy. Journal of Veterinary Science, 202(2), 292-296. https://doi.org/10.1016/j.tvjl.2014.08.008
Baird-Heinz, H. E., Van Schoick, A. L., Pelsor, F. R., Ranivand, L., & Hungerford, L. L. (2012). A systematic review of the safety of potassium bromide in dogs. Journal of the American Veterinary Medical Association, 240(6), 705-715. https://doi.org/10.2460/javma.240.6.705
Bhatti, S. F., De Risio, L., Muñana, K., Penderis, J., Stein, V. M., Tipold, A., Berendt, M., Farquhar, R. G., Fischer, A., Long, S., Löscher, W., Mandigers, P. J., Matiasek, K., Pakozdy, A., Patterson, E. E., Platt, S., Podell, M., Potschka, H., Rusbridge, C., & Volk, H. A. (2015). International Veterinary Epilepsy Task Force consensus proposal: Medical treatment of canine epilepsy in Europe. BMC Veterinary Research, 11, 176-191. https://doi.org/10.1186/s12917-015-0464-z
Caspi, D., Snel, J., Batt, R., Bennett, D., Rutteman, G., Hartman, E. G., Baltz, M. L., Gruys, E., & Pepys, M. B. (1987). C-reactive protein in dogs. American Journal of Veterinary Research, 48(6), 919-921. https://doi.org/10.2460/ajvr.1987.48.06.919
Ernst, R., Ogeer, J., McCrann, D., Cross, J., Strong-Townsend, M., Friis, H., Coyne, M., Clements, C., Drake, C., & Murphy, R. (2018). Comparative performance of IDEXX SDMA Test and the DLD SDMA ELISA for the measurement of SDMA in canine and feline serum. PLoS One, 13(10), e0205030. https://doi.org/10.1371/journal.pone.0205030
Feijoo, F., Ricart, M., Marina, L., Passeri, M., & Suraniti, A. (2020). Vómito, sialorrea y regurgitación como signos clínicos de convulsiones epilépticas focales autonómicas en tres perros. Revista Veterinaria, (41), 107-113. https://doi.org/10.19052/mv.vol1.iss41.10
Goiz-Márquez, G., Solís, H., Caballero, S., & Sumano, H. (2008). Epilepsia en perros. Veterinaria México Journal, 39(3), 279-321. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0301-50922008000300005&lng=es&tlng=es
Hammer, O., Harper, D., & Ryan, P. (2001). PAST: Paleontological Statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 4.
Han, W., Bailly, V., Abchandani, R., Thadhani, R., & Bonventre, J. (2002). Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney International, 62, 237-244. https://doi.org/10.1046/j.1523-1755.2002.00433.x
Harjen, H. J., Nicolaysen, T. V., Negard, T., Lund, H., Sævik, B. K., Anfinsen, K. P., Moldal, E. R., Zimmer, K. E., & Rørtveit, R. (2021). Serial serum creatinine, SDMA and urinary acute kidney injury biomarker measurements in dogs envenomated by the European adder (Vipera berus). BMC Veterinary Research, 17, 154. https://doi.org/10.1186/s12917-021-02851-8
Ichimura, T., Asseldonk, E. J., Humphreys, B. D., Gunaratnam, L., Duffield, J. S., & Bonventre, J. V. (2008). Kidney injury molecule–1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. Journal of Clinical Investigation, 118(5), 1657-1668. https://doi.org/10.1172/JCI34487
Joyce, D., Matz, L., & Saker, B. (1985). Renal failure and upper urinary tract obstruction after retrograde pyelography with potassium bromide solution. Human Toxicology, 4(5), 481-490. https://doi.org/10.1177/096032718500400504
Kielstein, J., Salpeter, S., Bode-Boeger, S., Cooke, J., & Fliser, D. (2006). Symmetric dimethylarginine (SDMA) as endogenous marker of renal function: A meta-analysis. Nephrology Dialysis Transplantation, 21, 2446-2451. https://doi.org/10.1093/ndt/gfl292
Lippi, I., Perondi, F., Meucci, V., Bruno, B., Gazzano, V., & Guidi, G. (2018). Clinical utility of urine kidney injury molecule-1 (KIM-1) and gamma-glutamyl transferase (GGT) in the diagnosis of canine acute kidney injury. Veterinary Research Communications, 42(2), 95-100. https://doi.org/10.1007/s11259-018-9711-7
Lu-Steffes, M., Pittluck, G. W., Jolley, M. E., Panas, H. N., Olive, D. L., Wang, C.-H., Nystrom, D. D., Keegan, C. L., Davis, T. P., & Stroupe, S. D. (1982). Fluorescence polarization immunoassay IV. Determination of phenytoin and phenobarbital in human serum and plasma. Clinical Chemistry, 28(11), 2278-2282. https://doi.org/10.1093/clinchem/28.11.2278
Metairon, S., Zamboni, C., Kovacs, L., Genezini, F., Santos, N., & Vilela, E. C. (2009). Analysis of elements in human blood of patients with chronic kidney disease using neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry, 282(1), 81-84. https://doi.org/10.1007/s10967-009-0272-7
Miguel, C., Giménez, M., & Meder, A. (2021). Neutrophil gelatinase-associated lipocalin (NGAL): Biomarker of acute kidney injury in dogs. Brazilian Journal of Veterinary Research and Animal Science, 4(2), 2490-2503. https://doi.org/10.34188/bjaerv4n2-076
Mishra, J., Dent, C., Tarabishi, R., Mitsnefes, M. M., Ma, Q., Kelly, C., Ruff, S. M., Zahedi, K., Shao, M., Bean, J., Mori, K., Barasch, J., & Devarajan, P. (2005). Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. The Lancet, 365(9466), 1231-1238. https://doi.org/10.1016/S0140-6736(05)74811-X
Muñana, K. (2013). Update seizure management in small animal practice. Veterinary Clinics of North America: Small Animal Practice, 43(5), 1127-1147. https://doi.org/10.1016/j.cvsm.2013.04.008
Nabity, M., & Hokamp, J. (2023). Urinary biomarkers of kidney disease in dogs and cats. Veterinary Clinics: Small Animal Practice, 53, 53-71. https://doi.org/10.1016/j.cvsm.2022.07.006
Noemi, I. (1999). Eosinofilia y parasitosis. Revista Chilena de Pediatría, 70(5), 435-440. https://doi.org/10.4067/S0370-41061999000500013
Parra, M., Cabezas-Herrera, J., & Cerón, J. (2005). Comparación de dos métodos para el aislamiento y purificación de la proteína C-reactiva (CRP) canina. Revista Científica de la Universidad de Murcia, 21, 139-149. https://revistas.um.es/analesvet/article/view/2791
Pérez-Sánchez, A., Perini-Perera, S., Del-Angel-Caraza, J., & Quijano-Hernández, I. (2023). Correlation of renal function biomarkers in the first diagnostic approach to canine chronic kidney disease. Journal MVZ Córdoba, 28(1), e2782. https://doi.org/10.21897/rmvz.2782
Perini, S., Pérez, A., Del-Ángel, J., Quijano, A., & Recillas, S. (2021). Evaluation of chronic kidney disease progression in dogs with therapeutic management of risk factors. Frontiers in Veterinary Science, 5, 621084. https://doi.org/10.3389/fvets.2021.621084
Podell, M. (2013). Antiepileptic drug therapy and monitoring. Topics in Companion Animal Medicine, 28, 59-66. https://doi.org/10.1053/j.tcam.2013.06.009
Potschka, H., Fischer, A., Löscher, W., Patterson, N., Bhatti, S., Berendt, M., De Risio, L., Farquhar, R., Long, S., Mandigers, P., Matiasek, K., Muñana, K., Pakozdy, A., Penderis, J., Platt, S., Podell, M., Rusbridge, C., Stein, V., Tipold, A., & Volk, H. A. (2015). International Veterinary Epilepsy Task Force consensus proposal: Outcome of therapeutic interventions in canine and feline epilepsy. BMC Veterinary Research, 11(1), 177-189. https://doi.org/10.1186/s12917-015-0465-y
Rebar, H. (2003). Leucocitos en Períodos de Salud y Enfermedad. En Interpretación del hemograma canino y felino (pp. 11-19). The Gloyd Group.
Reyes-Uribe, E., Hernández-Bedolla, M., Salazar-Flores, J., & Torres-Sánchez, D. (2022). La proteína KIM-1, un biomarcador asociado a la enfermedad renal. Revista Huasteca, 10(19), 20-27. https://doi.org/10.29057/esh.v10i19.8213
Robaina, D., Bentancur, V., Feijóo, G., Damián, J. P., & Suárez, G. (2020). Validation and clinical application of a spectrophotometric technique for the determination of potassium bromide in canine serum for the control of epilepsy. Journal of Pharmacy & Pharmacognosy Research, 8(6), 515-524. https://doi.org/10.56499/jppres20.875_8.6.515
Segev, G., Palm, C., LeRoy, B., & Cowgill, L. (2013). Evaluation of NGAL lipocalin as a marker of kidney injury. Journal of Veterinary Internal Medicine, 27(6), 1362-1367. https://doi.org/10.1111/jvim.12180
Soni, S. S., Cruz, D., Bobek, I., Chionh, C. Y., Nalesso, F., Lentini, P., de Cal, M., Corradi, V., Virzi, G., & Ronco, C. (2009). NGAL: A biomarker of acute kidney injury and other systemic conditions. International Urology and Nephrology, 42, 141-150. https://doi.org/10.1007/s11255-009-9608-z
Thomas, W. (2000). Idiopathic epilepsy in dogs. Veterinary Clinics of North America: Small Animal Practice, 30, 183-206. https://doi.org/10.1016/S0195-5616(00)50009-6
Van Logten, M., Wolthuïs, M., Rauws, A., & Kroes, R. (1973). Short-term toxicity study on sodium bromide in rats. Toxicology, 1(4), 321-327. https://doi.org/10.1016/0300-483X(73)90038-3
Wasung, M., Chawla, L., & Madero, M. (2015). Biomarkers of renal function, which and when. Clinica Chimica Acta, 438, 350-357. https://doi.org/10.1016/j.cca.2014.08.039
Yokota, T., Omachi, K., Suico, M. A., Kojima, H., Kamura, M., Teramoto, K., Kaseda, S., Kuwazuru, J., Shuto, T., & Kai, H. (2017). Bromide supplementation exacerbated the renal dysfunction, injury and fibrosis in a mouse model of Alport syndrome. PLoS One, 12(9), e0183959. https://doi.org/10.1371/journal.pone.0183959
Zar, J. H. (2010). Biostatistical analysis (5th ed.). Prentice Hall.
Zheng, J. S., Jing-Nie, Zhu, T. T., Ruan, H. R., Xue-Wei, & Rui-Wu. (2019). Screening of early diagnostic markers of gentamicin-induced acute kidney injury in canines. Journal of Veterinary Research, 63(3), 405-411. https://doi.org/10.2478/jvetres-2019-0048
Acknowledgements/Disclaimers/Conflict of interest
We thank Florencia Barrios for her contributions in correcting the manuscript.
We would like to thank the Faculty of Veterinary for its financial contribution to the development of this work.
We, as authors, hereby declare that we have no conflict of interest, whether financial, professional, or personal, in relation to “Evaluation of potential kidney damage associated with the use of phenobarbital and potassium bromide in epileptic canines using biomarkers predicting early damage”.
Contribution note
1. Conceptualisation, 2. Data curation, 3. Formal analysis, 4. Funding acquisition, 5. Investigation, 6. Methodology, 7. Project administration, 8. Writing - original draft, 9. Writing - review and editing, 10. Resources, 11. Supervision.
Clarisa Brighenti has contributed to 1, 2, 3, 5, 6, 8, and 9. Alejandra Capelli has contributed to 3, 5, 6, 9, and 11. Walter Norbis has contributed to 2, 3, and 9. Claudia Della Cella has contributed to 1 and 4. Luis Delucchi has contributed to 1, 4, 6, and 10. Catherine Fagúndez has contributed to 1, 2, 3, 5, 6, 7, 8, 9, 10, and 11.
Data availability
The dataset supporting the results of this study is available in Supporting information and Brighenti Viera, C. (2023). Determinación de biomarcadores renales asociados a la administración de bromuro de potasio y fenobarbital en caninos con epilepsia idiopátic [Tesis de maestría inédita]. Facultad de Veterinaria, Universidad de la República.
Nota del editor
El editor José Manuel Verdes aprobó este artículo.